
The CE milestone comes after the company recently obtained a UKCA mark and signed an agreement with Medicina, a large healthcare distributor. It marks another achievement for the firm after securing funding in December. The certification opens up a market worth $1.3 billion by 2027.
Despite many complications for companies trying to export to Europe since Brexit, the E.A.T. team anticipate the high-impact nature of their device, together with its distribution agreements, will help it overcome trade barriers and see increased trade in a sector.
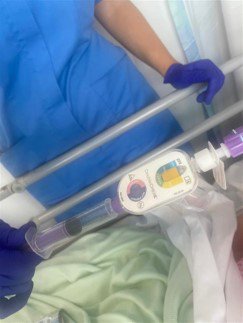
DoubleCHEK is an enteral feeding device that uses CO2 and pH indicators, coupled with a proprietary valving system, to help clinicians place naso/oro gastric tubes safely and quickly in any environment. Enteral feeding is used when patient cannot receive their nutrition orally.
Misplacement of feeding tubes can result in serious patient harm, and often happens because clinicians make decisions on placement with limited information. It is estimated that 3–5% of all NG tubes inserted are misplaced into the airway, and approximately 33% of those misplacements result in serious morbidity, such as pneumonia or pneumothorax, or even death. DoubleCHEK provides clinicians with critical information to avoid NG tube misplacement.
George Gallagher, founder and CEO, said: “We are pleased to now be able to affix the CE Mark to our DoubleCHEK device. The team has worked hard to achieve this milestone, which gives our shareholders confidence that their belief in DoubleCHEK was well placed. This achievement, together with our UKCA Mark and application to US FDA for the 510(k), means the regulatory outlook for DoubleCHEK is excellent.
The market opportunity for our product is now far greater and we are excited to now be able to take this patient safety technology to clinicians throughout Europe.”














